Therapeutic Products - Events Registration of Interest
Overview
Consultation on an exposure draft of the Therapeutic Products Bill and an associated consultation paper is underway. The Bill is intended to repeal and replace the Medicines Act 1981 and establish a new regulatory scheme for therapeutic products.
Why your views matter
We are holding focus meetings on specific topics to assist stakeholders to provide feedback on the regulatory proposals.
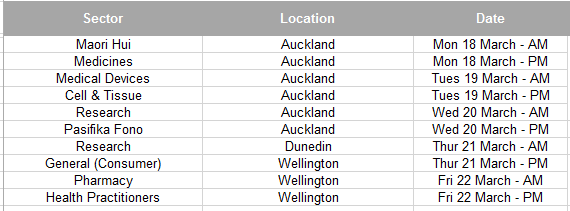
List of focus meetings we are proposing;
What happens next
Once registrations have closed, you will receive an email with event information.
Audiences
- Members of the public
- People affected by gambling harm
- Health sector
- Disability sector
- Ministry staff
- Gambling sector
- Mental health and addiction services
- Pharmaceutical companies
- Service providers
Interests
- Therapeutic products
- Assisted reproduction
- Medical devices
- Health information standards
- Emerging Health Technologies
- Gambling
- Mental health
- Substance use
- Pharmacy
- Research
- Cancer services
- Electives
- Change programme
- Technology
- Accommodation
- Travel
- Nutrition and physical activity
- Screening
- Pacific health

Share
Share on Twitter Share on Facebook